-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供して、未来を拓く学生たちの助けとなることを目指しています。
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供して、未来を拓く学生たちの助けとなることを目指しています。
-
Ansysは、シミュレーションエンジニアリングソフトウェアを学生に無償で提供して、未来を拓く学生たちの助けとなることを目指しています。
ANSYS ADVANTAGE MAGAZINE
DATE: 2020
Simulation for Regulation
By Bill Murray, President and CEO, and Dawn Bardot, Senior Program Manager Medical Device Innovation Consortium, St. Louis Park, U.S.A.
The Medical Device Innovation Consortium advances the use of simulation in the development and regulation of medical devices.
Twenty-first–century medical device development and clinical trial design must leverage evidence from computer modeling and simulation.
Computer modeling and simulation can revolutionize the field of medical devices, bringing innovative new treatments to patients faster and more safely. The Medical Device Innovation Consortium (MDIC) is working to advance the use of validated modeling and simulation in evaluation of regulated medical devices.
To provide state-of-the-art care for patients and keep pace with technology advancements, 21st-century medical device development and clinical trial design must leverage evidence from computer modeling and simulation. Historically, medical devices were evaluated based on three pillars of evidence: bench testing, animal studies and clinical trials. Computer modeling is now the fourth pillar of evidence, providing valuable insights early and often in device design and deployment. MDIC aims to identify and standardize modeling and simulation validation requirements so that computer simulations can become an integral source of evidence of medical device safety, efficacy and performance.
MDIC was formed in 2012 as a public–private partnership in which industry, government, nonprofit, patient groups and academia can come together to identify ways to bring medical devices to patients more safely, quickly and cost-effectively. To accomplish this overarching goal, MDIC established four project areas that focus on initiatives directly related to the clinical design, trial and process involved in bringing medical devices to market.
CURRENT ROLE OF MODELING AND SIMULATION
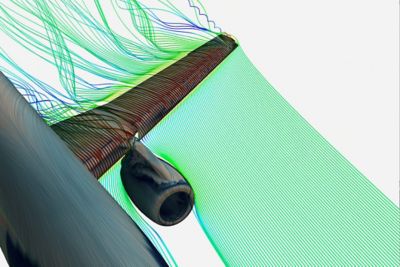
Currently, the medical device industry uses modeling and simulation tools in the initial proof-of-concept and prototyping phases. By creating these models earlier than ever before, engineers are able to evaluate candidate designs before building and testing prototypes. They can perform virtual tests early in the development process rather than waiting for prototype creation in a later development phase.
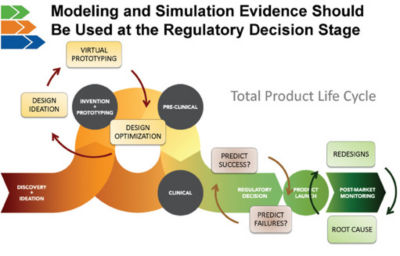
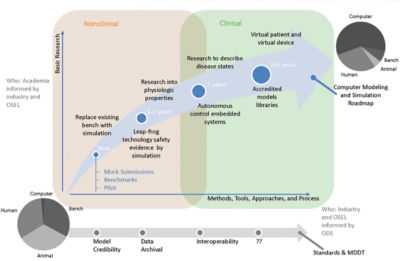
MDIC sees broad value in modeling and simulation. The consortium created the Computational Modeling & Simulation (CM&S) project to develop the tools and methods needed to extend the use of modeling and simulation throughout the total product lifecycle. The next big opportunity for computer models is at the regulatory decision phase of the lifecycle. MDIC builds confidence in these models’ validated, predictive capabilities so that they can be reliably used to make regulatory decisions. This will bring a return on investment through reduced reliance on animal studies and bench testing. Predictive simulations are the gateway to personalized medicine.
Modeling and simulation evidence can be used to make regulatory decisions instead of just for proof-of-concept and virtual prototyping.
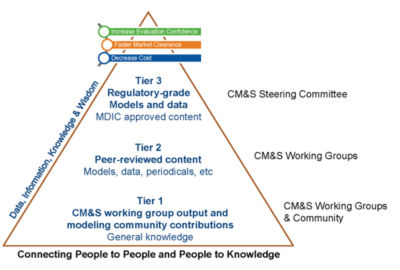
Structure of MDIC repository for data and models
The next big opportunity for computer models is at the regulatory decision phase of the lifecycle.
PRIORITIES FOR INCREASING USE OF MODELING AND SIMULATION
The CM&S project steering committee members share a vision of fostering medical innovation, assessing new and emerging technologies, and developing new ways of using clinical data to evaluate medical devices by conducting work in priority areas. This is achieved through working groups with member representation, including medical device manufacturers, the U.S. Food and Drug Administration (FDA), the National Institutes of Health (NIH), nonprofit groups and organizations with expertise in modeling and simulation. Priority areas include:
- Creating a framework to augment some clinical trials with virtual patients, paving the way for smaller, more cost-effective trials
- Identifying opportunities for simulation tools to gather data that cannot be captured by bench testing, particularly in the area of cardiovascular device design
- Developing models to establish the compatibility of implanted devices, such as pacemakers, with magnetic resonance imaging (MRI) by developing generic, regulatory-grade safety simulations that manufacturers can adapt to their specific needs
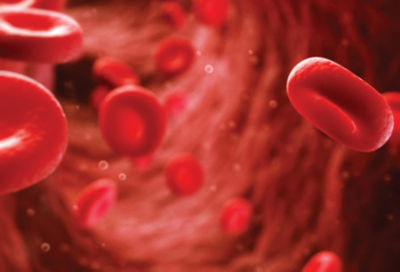
- Creating a simulation tool that can measure, quantitatively rather than qualitatively, the damage that can occur to blood cells when exposed to a medical device
- Constructing models of both basic healthy human physiology and diseased states to create, design and test new medical products to improve clinical outcomes
- Archiving data and resources on modeling and simulation to make them available to the entire medical device community
Road map for increasing the use of modeling and simulation evidence
In addition to fostering project specific initiatives and research, MDIC hosts events and gives presentations in national forums to build awareness of modeling and simulation and its importance. The organization is writing a white paper detailing the evolution of modeling and simulation in the medical device industry, current and future applications, barriers to using modeling and simulation in the regulatory process, and next steps. Simultaneously, the consortium is developing mock FDA submissions to produce representative examples of the use of computational modeling and simulation for actual regulatory submissions.
Ansys and MDIC
Ansys understands and embraces MDIC’s vision and has been an actively participating member of the consortium since its founding. Evolving from a product and technology tool provider to an innovator in healthcare simulation looking at physiological models, Ansys has played an important role in collaborating with MDIC’s CM&S project. Thierry Marchal championed the participation of Ansys in the MDIC. Marc Horner, Ansys healthcare industry technical lead, is a member of the Computational Modeling & Simulation Steering Committee and leads the blood damage working group. Horner is also a member of the RF heating and clinical trials working groups. The work performed in these groups will enable medical device developers to generate more groundbreaking ideas, test with greater confidence at lower cost, and bring devices to patients more safely and quickly.
Computer simulations can become an integral source of evidence of medical device safety, efficacy and performance.
References
[1] MDIC mdic.org
[2] Q&A with Marc Horner
mdic.org/spotlight-on-members-marc-horner/
Ansysができること
Ansysができること
お問い合わせ
お問い合わせいただき、ありがとうございます。
当社はお客様の質問にお答えし、お客様とお話できることを楽しみにしています。Ansysの営業担当が折り返しご連絡いたします。