-
-
Access Free Student Software
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
-
Connect with Ansys Now!
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Countries & Regions
Free Trials
Products & Services
Learn
About
Back
Products & Services
Back
Learn
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
Back
About
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Free Trials

Drug research and development (R&D) and manufacturing are continuously innovating to provide more effective medicines to patients in less time. The number of novel drugs approved annually by the U.S. Food and Drug Administration (FDA) has gradually increased over the past few decades, from 24 in 2008 to 55 in 2023. The process to get a drug approved is time- and cost-intensive. The time from initial drug discovery to full market approval can take 12 years and cost upward of $1 billion. With 21% of global pharmaceutical industry revenue spent on R&D, being first to market is of utmost importance for companies to receive a maximum return on investment (ROI) and fund new drug discoveries.
Pfizer is at the forefront of drug innovation. The company’s global portfolio includes medicines, vaccines, and many of the world’s best-known consumer healthcare products. Every day, Pfizer colleagues work across emerging and developed markets to advance wellness, prevention, treatments, and cures that challenge the most feared diseases of our time. As of Feb. 4, 2025, Pfizer has 115 therapies in the pipeline.
“Through the lens of simulation, we glimpse the future of medicine, where virtual realms become the crucible for innovation, allowing us to decode the barriers to process development and craft breakthroughs that change patients’ lives,” says Dr. Morgan Harris, MSAT CFD group lead at Pfizer.
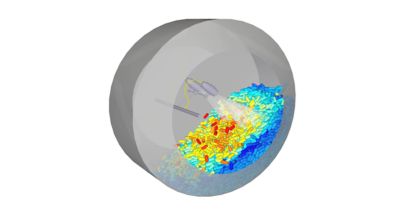
To verify its vessel and impeller library, Pfizer compared the simulation of a mixing tank with the performance of a real tank.
Simulation in R&D
While COVID-19 is thankfully now under control on a global scale, it was critically important for many pharmaceutical companies at the beginning of the pandemic to develop treatments in record time while maintaining safety. To save time, Pfizer’s quantitative systems pharmacology team simulated clinical trial outcomes in place of traditional Phase 2 trials. It used a viral kinetics model, which simulates virus replication and shows how the drug would inhibit that process. The model data helped inform the dose that the team would focus on in subsequent trials.
But another question arose: Would patients respond in five days, or did the medicine need more time to work? A 10-day treatment plan would mean more patients and a longer trial time while a five-day plan would cut that in half. The team simulated what-if scenarios and determined that just five days of treatment would work. After only about 12 months from initial discovery, the FDA approved Pfizer’s oral COVID-19 treatment for emergency use. While there were many brains working to expedite the approval process, the simulated clinical trial results helped accelerate the delivery of much-needed treatment to patients.
The team also uses simulated clinical trials to help develop a therapy for relapsed and refractory multiple myeloma, or plasma cell cancer. The team’s model simulates treatment periods that are longer than existing clinical observations. This enables experts to predict with confidence and test dose and regimen scenarios.
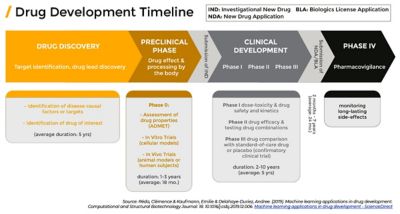
Source: Clémence Réda, Emilie Kaufmann, Andrée Delahaye-Duriez, Machine learning applications in drug development, Computational and Structural Biotechnology Journal, Volume 18, 2020, pages 241-252, https://doi.org/10.1016/j.csbj.2019.12.006
Simulation in Manufacturing
R&D is only part of the equation when it comes to developing pharmaceuticals. From small-scale clinical trials to full-scale manufacturing, the drugs need to be produced in varying amounts. The scale-up process of drug manufacturing is a delicate procedure that can lead to wasted resources and delayed time to market if done incorrectly. Pfizer uses simulation to help streamline many facets of scaling up.
Mixing
Mixing is extremely common throughout much of the manufacturing process. Mixing tanks with a poor design can cause uneven mixtures and other issues that undermine batch quality and efficacy. Manually iterating on blade and tank design or mixing processes is time-consuming and quickly increases costs. To verify its vessel and impeller library, Pfizer compared the simulation of a mixing tank with the performance of a real tank. The simulation blend time was exactly as the blend time performed in the lab. Unlike lab experiments, Pfizer knew the blend time at every location in the vessel, not at just a few discrete points measured by a pH probe.
Filtration
Hollow fiber tangential flow filtration (HF-TFF) cassettes are, as the name suggests, a method of filtration for biopharmaceuticals. Pfizer uses these to filter mRNA-lipid nanoparticles (LNPs). To optimize its LNP HF-TFF cassettes, Pfizer used simulation to extend the life cycles of the cassettes, increase the yield of viable mRNA-LNP, and develop the next version of TFF cassettes.
3D Printing
Pfizer wanted to develop a 3D-printed, optimized retentate (ultrafiltration) vessel that matched the performance of the pilot and manufacturing vessels. Using simulation, the company matched the blend time, mesomixing times, and micromixing times by fine-tuning the features of the 3D-printed retentate vessel. Using simulation improved the small-scale model (SSM) development for ultrafiltration and diafiltration (UF/DF) and improved the yield across the UF/DF step.
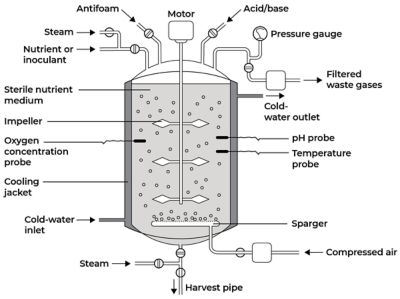
Example of a batch bioreactor design. Image adapted from Singh, Jagriti & Kaushik, Nirmala & Biswas, Soumitra. (2014). Bioreactors – Technology & Design Analysis.
The Future Is Now
Pfizer uses innovative technology to deliver medicines to people faster. Using simulation can help the company reduce clinical trial times, manufacturing, and time to market. These technologies are creating the next generation of medicine for the diseases that affect us most.
“At Pfizer, we envision a future where simulations are our guiding stars, illuminating the path to groundbreaking therapies, accelerating drug discovery, and revolutionizing patient care through precise, data-driven insights that transform possibilities into realities,” says Harris.
Simulated centrifuges running at low (left) and high (right) rotations per minute
Learn More
See how modeling and simulation address time, cost, and complexity challenges in healthcare.
Just for you. We have some additional resources you may enjoy.
“Through the lens of simulation, we glimpse the future of medicine, where virtual realms become the crucible for innovation, allowing us to decode the barriers to process development and craft breakthroughs that change patients’ lives.”
— Dr. Morgan Harris, MSAT CFD group lead, Pfizer
The Advantage Blog
The Ansys Advantage blog, featuring contributions from Ansys and other technology experts, keeps you updated on how Ansys simulation is powering innovation that drives human advancement.