-
-
Access Free Student Software
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
-
Connect with Ansys Now!
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Countries & Regions
Free Trials
Products & Services
Learn
About
Back
Products & Services
Back
Learn
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
Back
About
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Free Trials
ANSYS BLOG
November 30, 2023
Realizing the Potential of Digital Transformation in Drug Manufacturing
From initial discovery to launch, new drug development can take 10-15 years and can cost billions of dollars for a single drug. It is a very lengthy and expensive process. Growing costs and timelines mean increased challenges to get innovative treatments and medicines to those who need them most.
While there are many factors that contribute to the high cost and slow pace of getting a product to market — such as clinical trials and research and development — drug manufacturing and packaging processes add to the timeline and budget. Scaling up production, refinement, and packaging from lab to industrial level while maintaining patient safety, product quality, and sustainability efforts is a significant challenge.
The advantages for companies that successfully navigate drug manufacturing challenges are immense:
- Market exclusivity
- Patent protection
- Potentially billions in revenue
- Enhanced brand recognition
- Future research and development funding
But all of this is heavily dependent on being the first to market, and being first to market is aided by having efficient manufacturing processes. Traditionally, pharmaceutical companies use the trial-and-error method to refine recipes, equipment, and packaging. But this traditional approach requires numerous time-intensive physical iterations: manufacture the equipment, test a production line, measure the quality, and repeat until the desired quality is met. While widely accepted as a go-to strategy for scaling up, there is room for improvement.
Embracing digital transformation with computational modeling and simulation (CM&S) optimizes the process of scaling up drug manufacturing processes. CM&S accelerates timelines without compromising quality, advances equipment performance, reduces waste throughout the process, provides patients with much-needed medications, and increases margins and market position.
Simulation Supports Sustainability Efforts
Producing pharmaceuticals consumes vast amounts of water, energy, and ingredients to determine the right mixtures, equipment configurations, production setup, and packaging properties. The conventional approach of “build, test, repeat” is poorly tailored to helping companies achieve their sustainability goals. Whether scaling up and running production, identifying packaging needs, or managing operations and facilities, the conventional approach wastes significant amounts of resources.
CM&S solutions offer a more effective alternative. These solutions enable drug manufacturers to reduce waste by digitally optimizing configurations and equipment before using any physical resources.
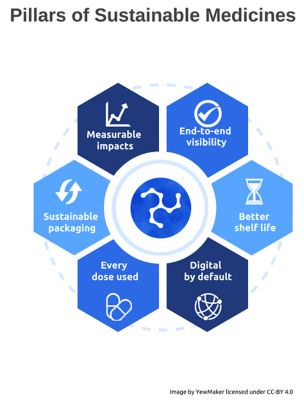
The Pillars of Sustainable Medicines set forth by the Sustainable Medicines Partnership, a non-profit action collaboration of 48 organizations.
Transform Pharmaceutical Upstream Processing
Pharmaceutical production begins with upstream processing. At this stage, manufacturers gather the required raw ingredients, including active pharmaceutical ingredients (APIs), water, solvents, and other chemicals. These components must be blended in specific proportions under tightly controlled conditions to ensure the quality and consistency of the final product. But this process is far from simple. Mixtures and environments must be exact, or expensive resources go to waste and companies lose their competitive advantage.
CM&S offer drug manufacturers a more efficient, sustainable approach. These technologies transform the scale-up and upstream production processes by enabling engineers to rapidly optimize mixing tank and bioreactor designs without relying on expensive, lengthy physical tests. Drug manufacturers can simulate the entire upstream process to ensure ideal production conditions at each stage and gain crucial insights into equipment performance.

Computational fluid dynamics (CFD) simulation of a mixing tank
Optimize Downstream Processing
The downstream chemical pharmaceutical and biopharmaceutical production processes consist of several essential steps in which product mixtures are refined, separated, and turned into finished tablets, capsules, and vaccines. Once full-scale production begins, navigating equipment failures and other disruptions are challenging. When technicians discover an issue, they have to shut down the machines, troubleshoot the problem, and make the necessary adjustments before restarting production. Because these disruptions often occur without warning, there is no opportunity to make those adjustments in real time before the product batch must be thrown out and production restarted.
In silico methods, however, deliver the understanding drug manufacturers need to scale and execute downstream drug production without the waste, high costs, and delays common to the conventional approach.
To meet the world’s evolving healthcare needs, pharmaceutical companies must find ways to accelerate the drug manufacturing process and get medicines to the patients more quickly. Modeling and simulation reduce costs and shorten the process of scaling up manufacturing efforts. Companies that continue to rely on conventional approaches to drug manufacturing delay their products’ much-needed arrival in the market.
Lean how in silico methods can help pharmaceutical companies throughout the drug manufacturing process.
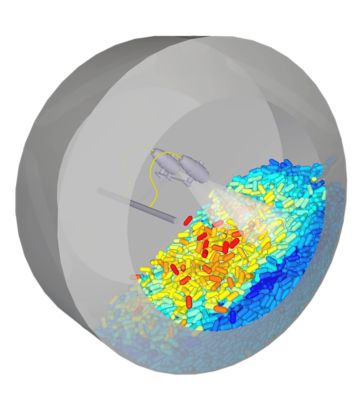
Simulating tablet coating in Ansys Rocky