-
-
Access Free Student Software
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
-
Connect with Ansys Now!
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Countries & Regions
Free Trials
Products & Services
Learn
About
Back
Products & Services
Back
Learn
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
Back
About
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Free Trials

The world’s leading pharmaceutical companies depend on Sartorius for high-quality, reliable equipment used in drug discovery. In turn, Sartorius relies on simulation to drive rapid, cost-effective, innovative product development.
You might not have heard the name Sartorius, but you’ve almost certainly used prescription drugs that were developed using its advanced solutions for the pharmaceutical industry. Headquartered in Göttingen, Germany, Sartorius is a €3.4 billion company with over 14,000 employees and 60 locations worldwide. The Sartorius Group is a leading international partner of life sciences research and the biopharmaceutical industry. With its products and solutions, Sartorius enables the development and production of new and better therapies as well as affordable medicine. From lab products and services to custom bioprocessing solutions, Sartorius supports the critical drug discovery efforts of every major biopharmaceutical company.
“One of the great things about being part of Sartorius is that our work has obvious importance and a demonstrated impact on society,” says Christian Becker, the company’s senior engineer for computer aided engineering (CAE) simulations. “As just one example, almost every COVID-19 vaccine was produced using equipment from Sartorius.”
“Running a single physical prototype of a bioreactor takes one month and costs up to €50,000. If we can replace iterative prototyping with simulation, then that’s a huge savings of time and money. And if we can replace a single 30-day test cycle with one week of iterative simulations, then obviously we can deliver solutions to customers much faster, while still having a high degree of confidence."
— Christian Becker, Senior Engineer for CAE Simulations, Sartorius
On the way to the marketplace, new drugs face tough challenges and create enormous costs for biopharmaceutical companies. Only one out of 10,000 drug candidates actually reaches the market, with an average cost of €2 billion and a development cycle, from discovery to regulatory approval, that can span 10 years. To protect those investments, drug makers need to ensure they’re working with partners that represent the leading edge in technology innovation, as well as uncompromising quality standards.
For biopharmaceuticals — a key area of focus for Sartorius — the challenges and risks are amplified. This class of drugs, which includes vaccines and insulin, consists of complex molecules that are obtained from living cells via cell culture processes. Their development costs, and their handling and quality-control requirements, are even greater than for other pharmaceuticals. Sartorius’ global leadership in this tough market segment is a testament to its own investments in world-class product development.

The world’s leading biopharma research teams rely on high-performing, high-quality solutions from Sartorius.
Taking Development to the Next Level With Ansys
Since 2019, simulation via Ansys has become a key component of the company’s product development strategy. Becker joined Sartorius that year to head up the company’s simulation efforts, bringing hands-on experience from the aerospace, automotive, and oil and gas industries.
“I think most people associate engineering simulation with those industries, and they’re surprised that a biopharma supplier would be leveraging such sophisticated product development methods,” notes Becker. “It’s evidence of how committed Sartorius is to providing advanced, leading-edge solutions to our customers.”
Together, the simulation team at Sartorius conducts multiphysics studies using Ansys Mechanical structural simulation software and Ansys Fluent fluid simulation software that reveals detailed performance of many of the company’s equipment — providing an “inside” view that would otherwise be impossible.
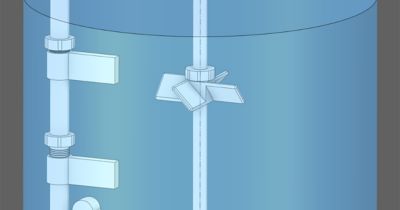
While many of Sartorius’ equipment offerings are constructed of single-use plastic parts, the simulation team for example helps perfect the design of the company’s single-use plastic bags that line bioreactors and other equipment during customer use.
“As our customers are developing drugs, they’re running 30-day batches, and then they see afterward if the product is fine or not,” explains Hazarika. “So during that month of continuous operation, the conditions must be tightly controlled. We need to understand exactly what’s happening inside the bioreactor. For example, are shear stresses being created? If so, how can we minimize them?”
“We not only need to design our single use bioreactors for structural strength and robustness to perform for 30 days, but we also need to optimize the behavior of the fluids inside them,” adds Maier. “After a certain time period, how will the viscosity change? How will the density change? With our visualizations enabled by Ansys, we can look directly into the reactor, which in many cases would not be possible in any other way.” Maier points out that the company’s bioreactors range from 15 ml to 2000 liter in working volume.
“Running a single physical prototype of a bioreactor takes one month and costs up to €50,000,” Becker states. “If we can replace iterative prototyping with simulation, then that’s a huge savings of time and money. And if we can replace a single 30-day test cycle with one week of iterative simulations, then obviously we can deliver solutions to customers much faster, while still having a high degree of confidence. We can’t eliminate experimental testing, but we can reduce it.”

Single-use bioreactors from Sartorius can range dramatically in size and volume, from 10 ml to 2,000 liter.
Tackling the Challenge of Increased Sustainability
“Sartorius is committed to maximizing cost and ease-of-use for our customers, as well as supporting sustainability,” explains Becker. “After a multiuse bioreactor has been used, it takes a lot of chemicals, hot water, and energy to clean the steel tank before another batch process can take place. We’ve addressed that by designing single-use plastic liners for our vessels, which are material or thermal recycled after use.
“Designing those bags with it’s stirring unit and connectors is a significant engineering challenge because you’re dealing with injection molding processes and complex part assemblies,” he continues. “And when you’re talking about plastic, there is a lot of nonlinear material behavior.”
“Engineering a product that includes both a reusable reactor and single-use liners for a customer application can easily cost over €1,000,000,” Becker says. “Ansys helps us predict how that product is going to perform in advance, accounting for nonlinear behaviors and other complexities.”
When Becker joined Sartorius, single-use plastics design was one of his first priorities, because they provide huge development speed and cost payback in addition to sustainability.
Single-use bioreactors from Sartorius can range dramatically in size and volume, from 10 ml to 2,000 liter. Biopharmaceutical development often requires 30-day batch processing, during which conditions must be tightly controlled to protect living cells.
A Healthy Outlook for the Future
Simulation has proven so valuable at Sartorius that Becker predicts his team will be doubling in size in the next five years or so. “Today, our three-person team is located in Gottingen, at the headquarters of Sartorius, but we want to get team members in India, France, and other regions more involved in simulation,” he says.
“In addition to growing the team our goal is to enable our colleagues to do their own preliminary studies in easy-to-use tools, like Ansys Discovery, then we’ll move those into Mechanical or Fluent to conduct deeper analysis,” Becker continues. “We need to increase our simulation bandwidth to keep driving innovation and answer our customers’ needs.”
With the rapid commercialization of COVID-19 vaccines and the emergence of mRNA technology, the global biopharmaceutical industry is experiencing incredible growth, which brings both opportunities and challenges for Sartorius’ customers. The company prides itself on helping customers drive product and process innovation via its own leading-edge solutions.
“Simulation with Ansys helps us maximize both development cost-effectiveness and speed, along with product confidence and reliability,” notes Becker. “As the benefits of engineering simulation become apparent, I expect more and more health care companies to adopt it as a standard product development practice.”
The Advantage Blog
The Ansys Advantage blog, featuring contributions from Ansys and other technology experts, keeps you updated on how Ansys simulation is powering innovation that drives human advancement.