-
-
Kostenlose Software für Studierende
Ansys unterstützt die nächste Generation von Ingenieur*innen
Studenten erhalten kostenlosen Zugang zu erstklassiger Simulationssoftware.
-
Verbinden Sie sich jetzt mit Ansys!
Gestalten Sie Ihre Zukunft
Stellen Sie eine Verbindung mit Ansys her, um zu erfahren, wie Simulation Ihren nächsten Durchbruch vorantreiben kann.
Länder und Regionen
Kostenlose Demoversionen
Produkte & Dienstleistungen
Lernportal
Über das Unternehmen
Back
Produkte & Dienstleistungen
Back
Lernportal
Ansys unterstützt die nächste Generation von Ingenieur*innen
Studenten erhalten kostenlosen Zugang zu erstklassiger Simulationssoftware.
Back
Über das Unternehmen
Gestalten Sie Ihre Zukunft
Stellen Sie eine Verbindung mit Ansys her, um zu erfahren, wie Simulation Ihren nächsten Durchbruch vorantreiben kann.
Kostenlose Demoversionen
ANSYS BLOG
February 6, 2020
Exploring the Important Challenges of Medical Device Design
To aid patients suffering from various medical conditions, biomedical engineers and medical professionals work tirelessly to create and improve medical devices designs.
With strict regulations and a lengthy development timeline, how do these practitioners make the necessary improvements to their designs? And how do they prepare the next generation for this task?
The answer is to teach and understand the theory of design, from concept to market, using a medical device database.
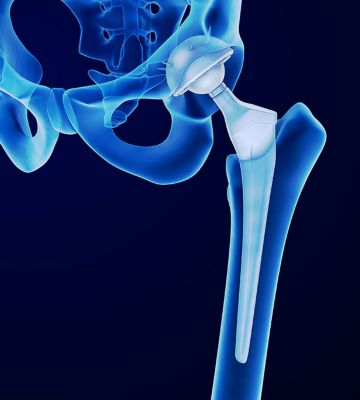
Databases can help the design of future hip implants.
How to Ensure Medical Device Designs are Ready for FDA Approval
Medical device design is a lengthy process that takes three to seven years to get from concept to market — in accordance with FDA regulations.
Once engineers start the approval process, it can be very difficult to make changes to the design — even when a further improvement has been discovered. Therefore, it is important to ensure all design possibilities have been considered before starting the regulatory process.
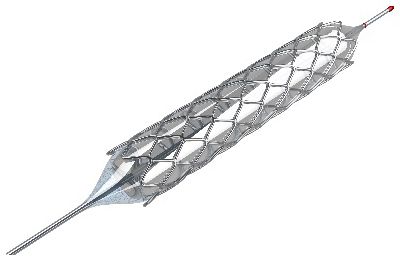
By understanding how blood flows through the heart, a stent can be properly designed to ensure proper heart function.
But, how do engineers account for every design iteration without dramatically extending their market timeline? Simulation is one way to refine aspects of medical device designs in less time.
For example, consider an implant — like a coronary stent. The heart undergoes significant structural changes as it pumps blood through its structure. By accurately modeling the impact of fluid-structure interactions, engineers can make important design decisions to improve the device early in the development cycle.
Engineers can also parameterize the simulation to test for various design possibilities. Once they have assessed various design iterations, they can be more confident about sending their medical devices for FDA approval.
Medical Device Database Enables Biomaterial Education
Before a stent can be manufactured, engineers must understand the fundamentals of biomaterials.
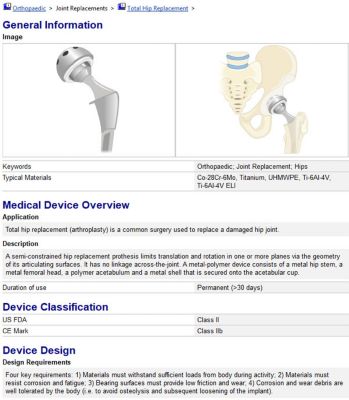
A device record of a hip implant from the medical device database within ANSYS GRANTA EduPack. Students can search for devices via FDA classifications, materials used and other design parameters.
Understanding how materials behave is a critical component of any product design. By adding the constraint of biocompatibility, the engineer’s job went from tricky to complicated.
Biomedical engineering instructors need to ensure that students fully comprehend the complexity of device design. To aid in this effort, they can use the medical device database within Ansys GRANTA EduPack. The database helps integrate the discussions of materials and devices within the classroom.
The database contains records for chemical elements, various materials and 50 devices. Each device record will include details such as its material components, classification and design details.
By taking advantage of the selection and exploration capabilities already within GRANTA EduPack, the medical device database will enable students to understand the complex nature of designing these products.
Any and all ANSYS, Inc. brand, product, service and feature names, logos and slogans such as Ansys and Ansys GRANTA are registered trademarks or trademarks of ANSYS, Inc. or its subsidiaries in the United States or other countries.