Ansys Blog
March 10, 2020
Chemical Kinetics: All You Need to Know
Consumers expect their products to be green, long-lasting and biocompatible. As a result, engineers will need to dust off their old chemistry textbooks.
That is because chemical kinetics and thermodynamics can affect the performance of almost any product. It’s active in the atmosphere, power generation, manufacturing, microelectronics and materials processing.
It’s also responsible for the rust and calcification that reduces the life of our designs.
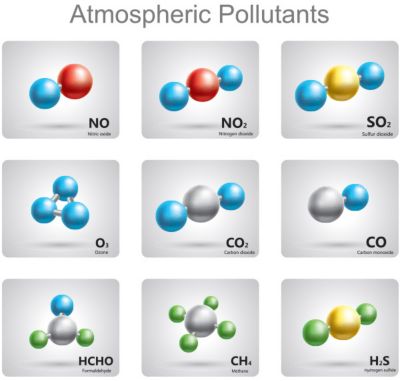
Using chemical kinetics and thermodynamics,
engineers reduce the number of
pollutants created by their designs
Even our planet — and every creature on it — are biochemical factories. So, chemical kinetics and thermodynamics help determine if a product is biocompatible and pollutant-free.
What is Chemical Kinetics?
Chemical kinetics tell us the speed at which chemical species transform into new substances by breaking and reforming their molecular bonds. In other words, it studies the rates and processes of chemical reactions.

Chemical kinetics studies the speed of reactions while thermodynamics
studies if the reaction will happen and in which direction
It should be noted that chemical kinetics differ from the thermodynamics of chemistry. The kinetics determines how and how fast you get there, while thermodynamics determines the direction you are going and where you eventually end up.
Think of it this way: if the chemicals are in thermodynamic equilibrium there will be no reaction — even if the kinetics indicate that reaction pathways exist.
What is Gibbs Free Energy?
Everything has potential energy. An apple in the tree has a potential to fall. When it falls, its gravitational potential energy is converted into kinetic energy.
You can think of the thermodynamics of chemistry the same way. Gibbs free energy, also known as chemical potential energy, tells you if chemical species will transform into other species.
Each chemical mixture has a different Gibbs free energy depending on its temperature, pressure and composition. Knowing the potential value for each species in a chemical reaction enables engineers to determine if and how they will react.

Gibbs free energy can be used to
assess the ability of a reaction
to proceed in either direction.
If the potential energy of the products is larger than the reactants, there is a barrier for that reaction to take place. If the potential energy of the products is less than the reactants, then the reaction will happen.
Just like the apple falling from the tree, nature wants to move the reaction to a point that minimizes potential energy.
Therefore, if an engineer wanted to push an equilibrium reaction to produce a certain product, they could manipulate the chemical potential of their chemical mixture by altering the temperature, pressure and species concentrations.
What is a Rate Law and How Does it Relate to Reaction Order?
Rate laws and reaction orders describe elementary chemical reactions.
These reversible reactions may involve:
- A single molecule decomposing into new species
- Two molecules/atoms colliding, and rearranging to form new species
- Two molecules/atoms colliding with the influence of a third body to form new species
The rate of this reaction is
dependent on the concentration
of red and yellow molecules
The reaction order refers to the number of chemical species involved as reactants.
Unimolecular reactions, where a molecule falls apart, has a reaction order of one. Reactions that need two molecules/atoms to collide have an order of two and so on.
For non-elementary (often called “global”) reactions, the reaction order may be determined empirically.
zA + yB + … + aZ = Products
Rate = k ∙ [A]z ∙ [B]y ∙ … ∙ [Z]a
The rate is dependent on a constant (k), the concentrations of the reactants ([A], [B] and [Z]) and the reaction order (z, y and a). The value of k can be determined computationally or empirically. Its units are based on the balanced equation of the chemical reaction and will change depending on the reaction order. The easiest way to remember them is to note that the units of the rate are always the same.
It’s impractical to determine the value of k for every reaction, either by measurement or computational molecular dynamics. Fortunately, engineers can infer the speed of unknown reactions based on a library of rate values for known reactions. So, by studying and classifying elementary reactions, scientists have developed rate rules for estimating other reactions.
Other factors that can affect the reaction rate include:
- Temperature
- Pressure
- Light (for photolytic reactions)
- Electromagnetics (for plasma or electrochemical reactions)
- The state of the chemical species: Solid, liquid or gas
- Presence of catalysts
- Molecular size and structure
- Surface coverage
- Particle size
What is a Global Reaction?
Often, chemical engineering textbooks will list a global reaction as they would elementary reactions:
zA + yB + … + aZ = Products
In reality, this reaction could have thousands of elementary sub-reactions happening in stages. The overall reaction — when all the elementary reactions go to completion — can then be simplify into the A, B, C and D components above.
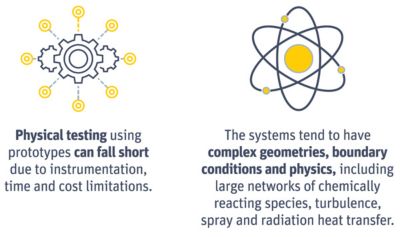
Physical testing and complex geometries make it difficult to
optimize complex reactions. However, simulation can simplify
the assessment of these systems
Some of these sub-reactions might not be dependent on pressure or temperature. Others might be quite complex, needing three or more species to collide before they react. Others can be sped up by introducing a catalyst to force the system to prefer certain reaction pathways.
Nonetheless, each of these sub-reactions can affect the rate of the overall reaction in various ways. Textbooks can teach engineers to combine elementary reactions to make sense of the overall reaction. However, when you have hundreds of species and thousands of reactions, the process is too complex for the average human to manipulate.
Traditionally, engineers were forced to determine the rate of complex reactions experimentally. These days, it is easy to use simulation to study these reactions in order to find the optimal temperature, process recipe or pressure to shift the system towards specific dominant pathways.
How Can Engineers Use Chemical Kinetics and Thermodynamics to Design Better Products?
To understand how chemical kinetics can optimize products, let’s look at a real-world example such as reducing engine emissions.
The conversion of fuel into carbon dioxide, water and various particles powers everyday life. However, the greenhouse and health effects of the end products can’t be ignored.
Through the use of chemical kinetics and thermodynamics, engineers can control how the fuel burns to reduce the release of certain pollutants.
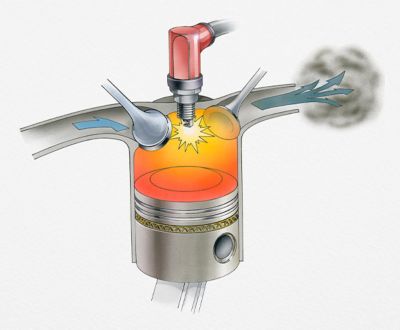
Chemical kinetics and thermodynamics
can help engineers design
better engines and reactors
The key is to control the temperature, pressure and air mixtures in the engine so the processes that produce the most energy dominate — with the least pollutants.
The challenge is that gasoline isn’t a monolithic chemical species. It’s made up of a distribution of substances that can vary in concentrations based on location, season and manufacturing processes. As a result, engineers need to design the engine, so it operates with a variety of fuels.
There are thousands of reactions at play within the engine, making it a complex system. Therefore, the best way to control which processes dominate is to use simulation.
Simulations can assess all the potential chemical reactions by including them in a computational fluid dynamics (CFD) simulation of the engine. Engineers can then use these simulations to tweak and optimize the designs until the target processes dominate.
Engineers can also add other aspects of the engine to the simulation to study engine efficiency tradeoffs, fuel effects, heat transfer and more. As a result, they are able to optimize a plethora of chemical reactor designs.
To learn more, read about Ansys Chemkin-Pro.
Any and all ANSYS, Inc. brand, product, service and feature names, logos and slogans such as Ansys, Ansys Chemkin and Ansys Chemkin-Pro are registered trademarks or trademarks of ANSYS, Inc. or its subsidiaries in the United States or other countries.