-
-
Software gratuito per studenti
Ansys potenzia la nuova generazione di ingegneri
Gli studenti hanno accesso gratuito a software di simulazione di livello mondiale.
-
Connettiti subito con Ansys!
Progetta il tuo futuro
Connettiti a Ansys per scoprire come la simulazione può potenziare la tua prossima innovazione.
Paesi e regioni
Customer Center
Supporto
Partner Community
Contatta l'ufficio vendite
Per Stati Uniti e Canada
Accedi
Prove Gratuite
Prodotti & Servizi
Scopri
Chi Siamo
Back
Prodotti & Servizi
Back
Scopri
Ansys potenzia la nuova generazione di ingegneri
Gli studenti hanno accesso gratuito a software di simulazione di livello mondiale.
Back
Chi Siamo
Progetta il tuo futuro
Connettiti a Ansys per scoprire come la simulazione può potenziare la tua prossima innovazione.
Customer Center
Supporto
Partner Community
Contatta l'ufficio vendite
Per Stati Uniti e Canada
Accedi
Prove Gratuite

Developing medical devices is a complex and high-stakes process, and precision, safety, and performance are non-negotiable. The road from initial concept to market-ready product is fraught with challenges, particularly when integrating advanced technologies like optical systems. Optical components are crucial in many medical devices, from diagnostic imaging tools like endoscopes and optical coherence tomography (OCT) systems to therapeutic lasers used in surgeries. These systems need to perform flawlessly in the most demanding conditions, and engineers must ensure they meet both functional and regulatory requirements.
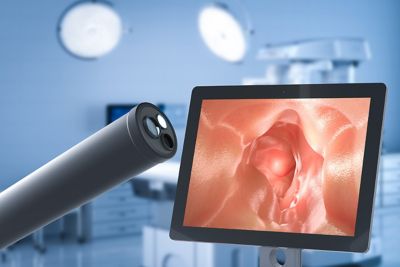
Example of an endoscope
The Complexities of Medical Device Development
Medical device development is inherently complex due to the key factors below.
Stringent Regulatory Requirements
Medical devices are subject to strict regulations from bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations ensure the safety, quality, and effectiveness of devices and require manufacturers to provide extensive documentation and evidence of performance before approval.
For optical systems, this means rigorous testing to ensure that they meet safety standards and deliver consistent, high-quality results.
Design and Integration Challenges
Modern medical devices often incorporate multiple technologies working in harmony. Optical, mechanical, electrical, and software systems must be integrated into a compact, functional unit. In particular, optical systems face challenges such as miniaturization, alignment, and precision. Even small deviations in the design of optical components can result in significant performance issues such as image distortion or misalignment, which could ultimately impact patient outcomes.
Cost and Manufacturing Considerations
Designing and manufacturing high-quality optical components can be costly. The challenge is to achieve the required performance standards while keeping production costs within budget. In addition, medical devices need to be manufactured at scale, meaning that optimizing the production process for consistency and cost-effectiveness is crucial.
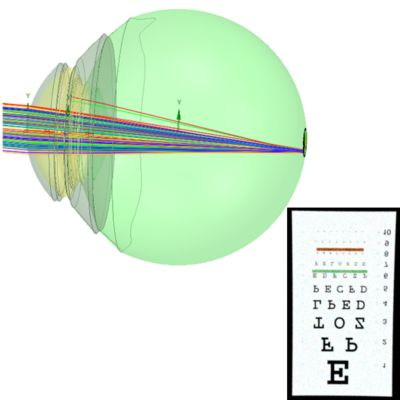
Modeling the human eye and designing presbyopia correction
A smart band heart rate sensor
Time-to-Market Pressures
The medical device market is highly competitive, and manufacturers are under pressure to deliver innovative solutions as quickly as possible. However, this urgency must be balanced with the need for thorough testing and validation to ensure that devices are safe and effective.
How Ansys Optics Solves These Challenges
The Ansys Optics product collection contains a powerful suite of optical simulation tools that enables engineers to optimize and test their optical systems early in the design process. By using Ansys Optics software solutions, manufacturers can address the challenges of medical device development more effectively, reducing the time, cost, and risks associated with bringing a new device to market.
Accelerate the Design Process With Simulation
One of the biggest challenges in medical device development is designing optical systems that meet performance, size, and cost constraints. Traditional design methods involve creating prototypes and physically testing them, which is time-consuming and costly. Ansys Optics tools change this by enabling engineers to simulate the entire optical system in a virtual environment. This enables rapid iteration of design concepts, which helps engineers quickly identify and address optical performance, alignment, and efficiency issues.
With tools like ray tracing and wave optics, Ansys Optics software can simulate how light interacts with optical components, enabling designers to optimize factors such as lens curvature, focal length, and light transmission without the need for physical prototypes. This speeds up the design process and ensures that the final product delivers the best possible performance from the outset.
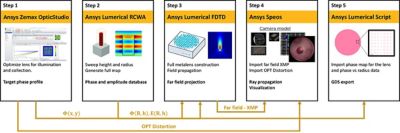
Ansys Optics endoscope application example
Multiphysics Integration: A Holistic Approach to Design
Medical devices are multidisciplinary by nature, and optical systems must work seamlessly with other subsystems like mechanical and electrical components. Ansys Optics offers a unique advantage by enabling multiphysics simulations, in which optical, thermal, and mechanical simulations are integrated into a single workflow. This holistic approach enables engineers to see how different subsystems interact with each other and how changes to one part of the system can impact the overall device performance.
For example, in a laser-based therapy device, engineers can simulate the optical behavior of the laser alongside its thermal effects and mechanical stress. This ensures that the optical components remain stable under operating conditions and that thermal management systems effectively dissipate heat, preventing damage to sensitive components.
Ansys Optics offers a unique advantage by enabling multiphysics simulations, in which optical, thermal, and mechanical simulations are integrated into a single workflow.
Achieving Reliable Performance With Thermal and Structural Analysis
Thermal and mechanical stresses can significantly affect the performance of optical systems. Ansys Optics offers integrated tools for thermal and structural analysis, enabling engineers to simulate the impact of temperature variations and mechanical deformations on optical components. This is particularly important in medical devices, as even small deviations can compromise the accuracy and effectiveness of treatments or diagnostics.
For instance, in a medical imaging system, thermal effects could cause optical components to expand or contract, leading to misalignment. Ansys Optics software can simulate these thermal variations and help engineers design systems that maintain alignment and performance across a range of operating temperatures. This ensures that the final product is robust and reliable, even in challenging conditions.
Streamlining Regulatory Approval With Validation Tools
Navigating the regulatory landscape is one of the most challenging aspects of medical device development. Regulatory bodies require extensive documentation and validation of medical devices before they can be approved for use. Ansys Optics tools help streamline this process by providing powerful validation capabilities that enable engineers to simulate and test optical performance to meet regulatory standards. By conducting virtual performance testing, engineers can ensure that their designs meet safety and quality standards before physical testing is even required.
Additionally, Ansys Optics software can provide detailed reports and documentation that can be used to support regulatory submissions, reducing the time and effort needed for compliance. This ensures that devices are not only safe and effective but also meet the necessary regulatory requirements for approval.
Reducing Costs and Optimizing Manufacturing
Medical device development can be expensive, particularly when it comes to manufacturing high-quality optical components. Ansys Optics helps reduce costs by enabling engineers to optimize designs for manufacturability. By simulating the optical system’s performance under different production conditions, engineers can identify potential manufacturing challenges early in the design phase and make adjustments to improve efficiency.
Moreover, simulation helps in selecting the most cost-effective materials and manufacturing techniques, ensuring that the final device is both affordable and of the highest quality. This can have a significant impact on both production costs and time to market.
See Ansys Optics Software in Action
To illustrate the impact of Ansys Optics, let’s consider a real-world example.
Skin cancer is the most common cancer in the United States, with approximately 5 million people treated every year. The European Union's Horizon Europe program funded the Intelligent Total Body Scanner for Early Detection of Melanoma (iToBoS) project to make early melanoma detection easier and more efficient. The platform includes a total body scanner and computer-aided diagnosis tool to integrate patient-specific data. The total body scanner will be powered by Optotune’s high-resolution cameras equipped with liquid lenses, providing unprecedented image quality of the whole body.
The iToBoS construction is similar to an MRI or CT scanner, with three camera modules that can move laterally and vertically. The lenses had to meet certain parameters, such as camera distance, scanner time, small field of view, and small depth of field. To test the different lens requirements, Optotune turned to Ansys Zemax OpticStudio optical system design and analysis software.
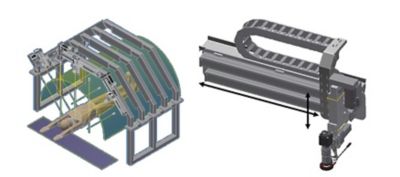
Rendering of the iToBoS (left) and the camera module (right)
Optotune needed to adjust the distance between the patient and the camera module (working distance) from 350 to 650 mm, with an intermediate distance of 420 mm. The nominal design of the scanner camera with liquid lens simulated in OpticStudio software was well above the target specifications. Still, the required optical quality of the iToBoS was very high.
Optotune created gravity-compensated (GC) lenses to compensate for the effect of gravity on extremely sensitive optics like the iToBoS. After simulating this worst-case scenario in OpticStudio software, the Optotune team determined that the lenses still meet the minimum acceptable parameters for all three distances.
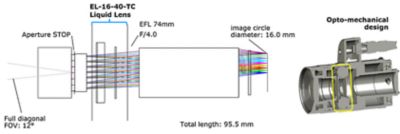
Camera module layout with performance characteristics (left) in Ansys Zemax OpticStudio software and the opto-mechanical design of the module with the yellow rectangle indicating where the liquid lens is housed (right)
Discover the Power of Ansys Optics
The path from concept to approval in medical device development is challenging, particularly when incorporating complex optical systems. Ansys Optics offers comprehensive solutions to these challenges, providing powerful tools for simulation, optimization, and validation that help accelerate the design process, ensure regulatory compliance, and reduce costs. By leveraging these capabilities, manufacturers can bring innovative, high-performance medical devices to market more efficiently and with greater confidence, ultimately improving patient outcomes and advancing the healthcare industry.
Whether you’re designing the next generation of imaging systems, laser therapies, or diagnostic tools, Ansys Optics is here to help you overcome the challenges of medical device development and turn your vision into reality. Watch the "Early Melanoma Detection: How Liquid Lenses Solve Optical Challenges" on-demand webinar to learn more.
The Advantage Blog
The Ansys Advantage blog, featuring contributions from Ansys and other technology experts, keeps you updated on how Ansys simulation is powering innovation that drives human advancement.