-
-
Access Free Student Software
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
-
Connect with Ansys Now!
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Countries & Regions
Free Trials
Products & Services
Learn
About
Back
Products & Services
Back
Learn
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
Back
About
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Free Trials
ANSYS BLOG
May 8, 2024
Democratizing In Silico Medical Device Testing
There are thousands of medical devices registered with the Food and Drug Administration (FDA), some being implanted orthopedic devices. Bone screws are the most common type of orthopedic implant because they are used in just about every joint replacement, plate fastening, and fixation device. While the typical bone screw has specific parameters set by regulatory authorities, medical device manufacturers are always looking for ways to create new, innovative screws that are safer for patients and more convenient for orthopedic surgeons. That’s where Numalogics comes in.
Numalogics helps small-, medium-, and large-sized medical device manufacturers design, test, and iterate on their devices using virtual models. This Canadian-based company was founded in 2010 by three orthopedic spinal surgeons whose goal was to provide insights to the biomechanics of human-device interactions. As a member of the American Society for Testing and Materials (ASTM) committee on medical and surgical materials and devices, an active contributor to the American Society of Mechanical Engineers (ASME) V&V40 committee, and a member of the Avicenna Alliance, Numalogics democratizes in silico medical device testing by creating easy-to-use applications with embedded ASTM and ISO standard tests. They use high-fidelity finite element analysis (FEA) models to create a library of different in silico tests so medical device manufacturers can understand the behavior and performance of their implants under modeled biological system (biofidelic) loading conditions. To establish credibility of their computational models, specifically for ASTM F543-A3: Test Method for Determining the Axial Pullout Strength of Medical Bone Screws, Numalogics joined forces with Ansys and Sawbones, a leading provider of bone surrogate materials.
Bone In, Please
Designing, prototyping, and conducting experimental testing of the screws is a frequent challenge for medical device manufacturers and can take months.
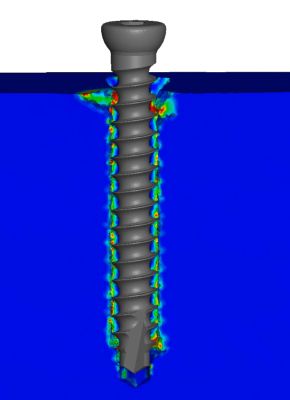
This wastes up valuable time and resources that could otherwise be spent somewhere else or delay a device’s submission to the FDA, therefore slowing down entry to market or inhibiting even engaging in such projects. Numalogics aims to shorten timelines and reduce R&D costs by replicating the ASTM standard that is used to test the performance of orthopedic bone screws with their virtual models. But before they could model the screws, they needed the other half of the equation: bone.
Cadaver bone might seem like the logical option for testing, but it’s not. Bone density varies wildly depending on sex, age, diet, previous medical conditions, etc., so there would be no way to guarantee consistency between the mechanical properties of the bone from test to test. It’s also not easy to acquire cadaver bone. So, Numalogics turned to Sawbones.
Sawbones creates a bone surrogate material that cuts, drills, and feels like real bone; is recognized by the FDA; and conforms to ASTM F-1839-08: Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices, making it the perfect base for Numalogics to test their models. They offer a variety of models that cover the entirety of the skeletal system and even have veterinary models for dogs, cats, and horses. Companies all over the world use their products to test orthopedic devices and provide hands-on learning to aspiring medical professionals. The rigid polyurethane foam block offers a standard way to evaluate the performance of orthopedic screws, as they offer consistent mechanical properties and enable performance comparisons between different screw designs.
“We virtualized the Sawbones material in a finite element model,” says David Benoit, Biomechanical Simulation R&D Specialist at Numalogics. “The material's mechanical behavior was characterized through extensive experimental testing across various loading modes, which enabled us to incorporate all material properties into the model. The credibility of the model was then evaluated by conducting a validation study using real orthopedic screws.”
An obvious but complex feature of a bone screw is its anchoring performance once fixed in the bone. Throughout its entire life cycle, regardless of bone density, the screw should not move. Numalogics used 17 different screws from five implant manufacturers and conducted experimental screw pullout testing for all of them. Then they simulated the screw pullout tests and compared the physical results to the predictions of the models. The numerical model showed excellent reliability in predicting the maximum pullout force of the screw.

Physical experimental testing of a bone screw versus the FEA testing of a bone screw (section view)
No Loose Screws Here
The success of the validation study led Numalogics and Sawbones to create an app that democratizes the use of simulation by making it easier to test bone screws in a virtual environment. Endpoint™, launched in early 2024, is a cloud-based app that enables engineers to easily test orthopedic screw designs using Numalogics’ latest computational modeling and simulation techniques and obtain results quickly, even before the first physical prototype can be manufactured.
“You only need the 3D model of the screw to run the simulation, which takes just a few minutes, to evaluate its anchoring performance,” says Benoit. “The model is ideal for conducting experimental design studies and converge towards the most optimal design while significantly reducing the time and resources required for prototyping and experimentally testing new designs.”
For now, to assist with usability and understanding, customers submit their computer-aided design (CAD) files and testing requirements to Sawbones and Numalogics engineers run the simulations in the app for them. The app automatically creates the geometry in Ansys SpaceClaim 3D modeling software then creates the model in Ansys Mechanical structural simulation software and runs the simulation using Ansys LS-DYNA explicit simulation software. The automation is handled via a dedicated ACT software extension, and they use the Ansys Data Processing Framework (DPF) from the PyAnsys code library for results visualization.
Eventually users will be able to independently upload their CAD files, specify a few simple parameters such as Sawbones’ block density and screw insertion depth, then let the app do the rest. And not only will this version of the app provide test results, but it will also provide the user with more control to gain insight to the mechanism of bone failure — something very important to optimizing and understanding bone screws.
“When you do experimental testing, it's hard to discover the reason of the failure or the failure mechanism inside the block of foam,” says Loïc Degueldre, Simulation Automation R&D Manager at Numalogics. “In the numerical simulation, we provide an animation of the failure. So, you can see what's really happening inside the bone and compare mechanisms of the failure between different designs.”

Workflow of the Numalogics and Sawbones app, Endpoint™
Validated, Verified, and Accepted
While this app will help many medical device manufacturers design new screws, it’s just the tip of the iceberg for the benefits Numalogics can provide.
“The next step is to facilitate the results evaluation by the regulatory bodies for our customers,” says Benoit. “Our goal is to have simulation results be accepted as evidence to demonstrate the screws’ performance so that it can be used to reduce or replace experimental testing.”
“In democratizing this simulation tool when it is eventually available as a web-based app,” says Degueldre, “we will help screw manufacturers significantly reduce time and resources for evaluating new screw designs before going to market.”
Learn how simulation can help you with your medical device innovations.