-
-
Software gratuito per studenti
Ansys potenzia la nuova generazione di ingegneri
Gli studenti hanno accesso gratuito a software di simulazione di livello mondiale.
-
Connettiti subito con Ansys!
Progetta il tuo futuro
Connettiti a Ansys per scoprire come la simulazione può potenziare la tua prossima innovazione.
Paesi e regioni
Customer Center
Supporto
Partner Community
Contatta l'ufficio vendite
Per Stati Uniti e Canada
Accedi
Prove Gratuite
Prodotti & Servizi
Scopri
Chi Siamo
Back
Prodotti & Servizi
Back
Scopri
Ansys potenzia la nuova generazione di ingegneri
Gli studenti hanno accesso gratuito a software di simulazione di livello mondiale.
Back
Chi Siamo
Progetta il tuo futuro
Connettiti a Ansys per scoprire come la simulazione può potenziare la tua prossima innovazione.
Customer Center
Supporto
Partner Community
Contatta l'ufficio vendite
Per Stati Uniti e Canada
Accedi
Prove Gratuite
ANSYS BLOG
May 24, 2022
Selecting the Right Battery For Your Application Part 3: Common Secondary Battery Chemistries
In part one of this three-part series, we discussed the most important metrics for battery selection. One thing to remember about battery selection is that it is essentially about managing tradeoffs. You trade off some battery metrics in order to gain in others — for example to gain power density, you may have to trade off energy density.
In this and a previous blog post, we discuss one of the most important aspects governing battery selection: battery cell chemistry. Many important cell properties such as energy density, flammability and safety, available cell constructions, temperature range, and shelf life are dictated by battery chemistry.
Applications with occasional intermittent use such as a smoke alarm, toy or flashlight, disposable applications, or when charging becomes impractical, warrant the use of a primary battery. Hearing aids, watches, greeting cards, and pacemakers are good examples. If the battery is to be used continuously and for long stretches of time, such as in a laptop, a cell phone, or a smartwatch, a rechargeable battery (secondary battery) is more suitable.
Let’s take a look at the most common secondary battery chemistries available.
1. Lithium-ion Batteries
Lithium is the lightest metal in the periodic table and has a specific capacity of 3860 mAh/g compared to Zinc at 820 mAh/g (battery capacity is what gives our devices talk time or run time). Lithium also has an electrochemical reduction potential of 3.045 V against 0.76 V for Zinc (i.e a lithium-based battery provides a battery voltage of 3 V or greater). The combination of these two properties results in very high energy densities for lithium-based batteries.
While lithium metal-based batteries could provide extremely high energy density, when these systems are charged there is the risk of dendritic growth that could penetrate the separator and create a short. We have all heard of the risks of battery explosions and thermal run away from the resulting temperature rise associated with lithium battery shorting. This risk is alleviated in lithium ion batteries, where the lithium is in a non-metallic form, and lithium ions move back and forth between the anode and cathode. Lithium-ion (Li-ion) batteries trade energy density (compared to lithium metal) to be able to charge in a relatively stable and safe manner.
Because of their excellent energy density and long cycle life (over 1000 cycles), these rechargeable systems are used in a wide variety of applications such as cell phones, laptops, plug-in hybrids, and electric vehicles. In the most common lithium-ion battery implementation, the anode is typically a graphite sheet, the cathode a lithium-cobalt oxide, and the electrolyte a lithium salt in an organic solvent.
Because lithium is highly reactive toward water, it cannot be used with aqueous electrolytes, unlike zinc. Organic electrolytes are commonly used — but these pale in conductivity compared to aqueous electrolytes like potassium hydroxide, zinc chloride etc., and limit the power output of lithium batteries (in order to get power, low battery resistance and high electrolyte conductivity are required). Due to the low conductivity of organic electrolytes, Li-based battery cell designs favor large surface area construction such as coin over button cells and jellyroll construction over the bobbin type in order to minimize internal resistance and enhance power capability. In other words, cell construction has to be relied on to increase power output for lithium-based batteries.
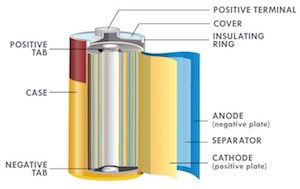
Figure 1: Jelly roll construction, optimized for power
A high cell voltage of 3.6 Volts or greater means fewer cells are needed for high voltage applications. For example, one Lithium cell can replace three NiCad or NiMH cells which have a cell voltage of 1.2 Volts. Unlike lead acid batteries, these can be deep cycled.
Disadvantages stem from the fact that lithium is so highly reactive that special safety electronics are required. Lithium ion batteries are also subject to transportation regulations (each airline passenger is restricted to carrying 2g of metallic lithium in primary batteries or 8g of rechargeable Li-ion). Safety issues include the possibility of thermal runaway when overcharged, abused, or used outside of the operating window.
2. Nickel-Metal-Hydride Batteries
Nickel metal hydride batteries use a nickel oxyhydroxide cathode, a hydrogen absorbing alloy as anode (replaces cadmium in the nickel-cadmium battery), and an alkaline electrolyte. They have good high-power capability, but cannot compete with lithium-ion batteries in terms of energy density. This is a 1.2-V battery system. They are resilient to both overcharge and discharge and can be operated between -30 to 75 C. While lithium-ion batteries have replaced this system in many consumer applications, nickel-metal-hydride still finds use in hybrid-electric vehicles, where it has seen more than 10 years of use. The active chemicals are also inherently safer than lithium-based systems, and don’t require the use of complex battery management systems. Nickel-metal-hydride batteries also compete with lithium ion in cost for applications like portable tools.
3. Lead-Acid Batteries
Lead-acid batteries have low energy densities, but are widely used in automotive starter batteries because of their ability to provide high surge currents inexpensively. No other battery system provides high power rate capability as cheaply and reliably as lead acid, and this system has been in use for 140 years! No wonder this battery system finds use in applications like golf carts, forklifts, UPS vehicles, electric scooters, and electric wheelchairs. Due to their low energy density, you will never see this system used in consumer applications.
4. Nickel-Cadmium Batteries
Nickel-cadmium batteries have lost significant market share to Li-ion and nickel-metal-hydride batteries since the 1990s, due to the toxicity of cadmium. They provide a voltage of 1.2 V, and use an alkaline electrolyte. Their ability to provide high discharge rates made portable electronic applications practical when they first came to market. Advantages of this system include high cycle life, wide temperature range, and their relatively high abuse tolerance. However, because of the toxicity of cadmium, these are being phased out for many consumer applications.
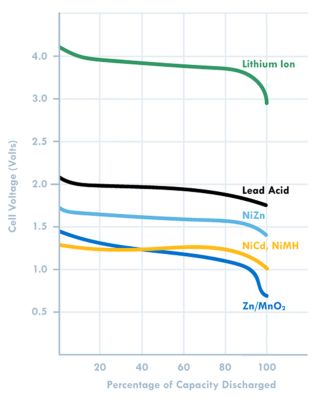
Figure 2: Voltage plot based on battery chemistry
There are a number of considerations that go into battery selection like performance, cost, safety, and shelf life among others. Using the most important battery metrics required by the application as a starting point helps narrow down the chemistry and design options.
Interested in improving your battery reliability? Request a quote here.