ANSYS BLOG
December 29, 2023
Maximizing Bioreactor Efficiency with Simulation
The process to make pharmaceuticals — whether pills, vaccines, or specialty medicines — is intense. Not only does the average drug take 10 to 12 years to develop, but it also costs billions of dollars. From research and development to manufacturing and distribution, every step must be carefully controlled to maintain patient safety. For biopharmaceuticals, bioreactors are at the heart of the process. Used to house organisms, these tanks are critical in manufacturing and safeguarding lifesaving drugs for patients. Drug manufacturers are always looking for new ways to optimize these processes, and the Ansys team recently developed a solution methodology to achieve that.

What is a Bioreactor?
Bioreactors can broadly be defined as vessels that house a biological catalyst to achieve a chemical transformation. They are designed to optimize growth and metabolic activity of an organism through biocatalysis using enzymes or cells of animals or plants. Bioreactors are different from chemical reactors in that they support and control biological matter, while chemical reactors only support chemicals. Because organisms are more sensitive and less stable than chemicals, bioreactors are more tightly controlled to prevent process deviations and contamination.
The reaction rate, cell growth, and process stability depend on the conditions inside the bioreactor. The following are a few examples of conditions that need to be closely monitored and controlled:
- Gas (e.g., air, oxygen, nitrogen, carbon dioxide) flow rates
- Temperature
- pH and dissolved oxygen levels
- Agitation speed and circulation rate
- Foam production

Types of Bioreactors
There are three main types of bioreactors: batch, continuous, and semi-continuous. The prospect of tank contamination has led many companies to use batch bioreactors in a closed process to minimize the chance of viruses or bacteria entering the tank. This is because contamination would require the company to discard the entire batch of drug, which could be worth millions of dollars in lost revenue.
Type | Advantages | Disadvantages |
Batch Process |
|
|
Continuous Process |
|
|
Semi-continuous or Fed-batch Process |
|
|
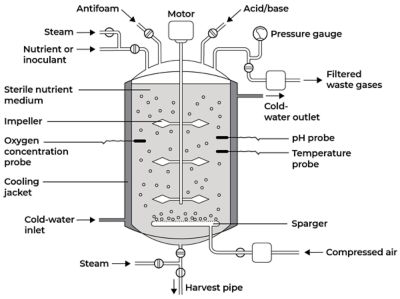
Example of a batch bioreactor design. Image adapted from Singh, Jagriti & Kaushik, Nirmala & Biswas, Soumitra. (2014). Bioreactors – Technology & Design Analysis.
Optimizing Bioreactor Design
Bioreactor processes are run for a period of weeks, and the amount of drug inside the tank at the end of the process represents how much is available to sell to patients. Therefore, manufacturers are continually striving to improve efficiency to maximize the value of each batch.
Biopharmaceutical manufacturers are not new to modeling the hydrodynamic environment inside their mixing tanks. They traditionally use computational fluid dynamics (CFD) to predict fluid flow patterns and how gas bubbles distribute throughout the tank and what that means for level of dissolved oxygen in the culture media. However, one major element of the manufacturing process is often ignored in modeling efforts: the relationship between the mixing patterns and the cells living inside the tank. To explore this, Ansys engineers developed a digital twin model of a bioreactor using Ansys Twin Builder and Ansys Twin Deployer. The digital twin model is composed of a CFD model coupled to a cell metabolic model.
A steady-state, 3D model of fluid flow and mass transport inside a bioreactor was developed and validated against experimental data. A design of experiments (DOE) was then conducted over the control space of the bioreactor to develop an understanding of how the rotation rate of the impeller and flow rate of oxygen impacted the mass transfer of oxygen into the liquid phase. The results of this DOE were exported as a functional mock-up unit (FMU).
A metabolic model representing the rates of cell growth, product production, tank pH, and sugar and oxygen consumption was developed using the Modelica programming library in Twin Builder. This model is composed of five ordinary differential equations — one for each parameter of interest. The metabolic model was validated against literature data. Note that the cell metabolic model runs quickly enough to not require a ROM.
The digital twin model was developed in Twin Builder, which consists of the mixing tank ROM connected to the Modelica model. A proportional-integral-derivative (PID) controller was tuned to ensure that the digital twin model would operate the mixing process to meet all performance requirements. In this case, the goal of the controller was to maintain the amount of oxygen in the liquid phase above 95% saturation.
The digital twin model was deployed using Twin Deployer. Using the resulting software development kit (SDK), a dashboard was created to facilitate visualization. This customizable dashboard provides a summary of the data streaming off process sensors and provides the current output of virtual sensors indicating other metrics of tank performance, e.g., concentration of cells in the tank and amount of drug product manufactured.
Ansys’ bioreactor digital twin model provides biopharmaceutical industry manufacturers with a more holistic understanding of bioreactor performance. Uniquely, Ansys’ solution includes an explicit relationship between the flow and mass transfer conditions in the tank and how those conditions impact the biological cells that are creating the drug. This enables drug manufacturers to continuously analyze and optimize tank performance based on current process parameters, ultimately leading to an increased tank yield.
To try it yourself, download a free trial of Ansys Twin Builder.