E-BOOK
-
-
학생용 무료 소프트웨어에 액세스하기
차세대 엔지니어에게 힘을 실어주는 Ansys
학생들은 세계적 수준의 시뮬레이션 소프트웨어를 무료로 이용할 수 있습니다.
-
지금 바로 Ansys에 연결하십시오!
미래를 설계하기
시뮬레이션이 다음 혁신을 어떻게 지원할 수 있는지 알아보려면 Ansys와 연결하십시오.
국가
무료 트라이얼
제품 및 서비스
학습하기
회사 정보
Back
제품 및 서비스
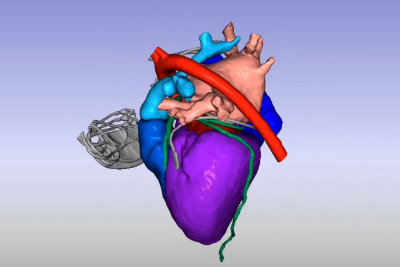
Due to their intricate design and lifesaving nature, developing cardiac rhythm management devices is challenging. Manufacturers must ensure the device functions as indicated throughout its intended lifetime without interfering with normal bodily functions. It must also withstand the harsh conditions of the human body and resist possible cybersecurity threats. Plus, no two patients are the same, with anatomies and diseased areas of the heart differing from person to person. Given their critical role in sustaining life, regulatory agencies around the world have very strict requirements that must be met before market entry.
Manufacturers can use computational modeling and simulation (CM&S) to help overcome the complex design and regulatory challenges associated with these devices while still maintaining the highest possible safety. CM&S enables device manufacturers to effectively iterate on designs while ensuring safety and reliability, accelerating the approval process and time to market.
Read the e-book to learn how CM&S can accelerate and optimize the cardiac rhythm management device development process.