-
-
Access Free Student Software
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
-
Connect with Ansys Now!
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Countries & Regions
Free Trials
Products & Services
Learn
About
Back
Products & Services
Back
Learn
Ansys empowers the next generation of engineers
Students get free access to world-class simulation software.
Back
About
Design your future
Connect with Ansys to explore how simulation can power your next breakthrough.
Free Trials
ANSYS BLOG
May 17, 2022
Selecting the Right Battery For Your Application Part 2: Common Primary Battery Chemistries
In part 1 of this three-part blog series, we discussed the most important metrics for battery selection. One thing to remember about battery selection is that it is essentially about managing tradeoffs. You trade off one feature in order to gain in another — for example in order to gain power density, you may have to reduce energy density.
In this and a subsequent blog post, we will discuss one of the most important aspects governing battery selection: battery cell chemistry. Many important cell properties such as energy density, flammability and safety, available cell constructions, temperature range, and shelf life are dictated by battery chemistry. So, let’s take a look at the most common primary battery chemistries available.
Common Primary Battery Chemistries
Zinc-carbon batteries have been around for more than 100 years. These are low cost and available in many shapes and sizes. However, they have lost market share to newer chemistries in the past few decades. They are still used in low drain intermittent use applications like remote controls, flashlights, and clocks.
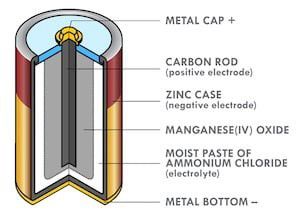
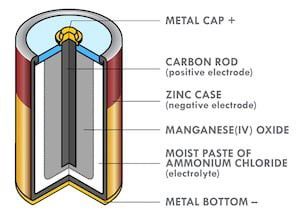
Figure 1: Cross-section of a zinc–carbon battery.
Alkaline zinc batteries were invented by Lewis Urry while he was employed by Eveready Battery Company. They provide higher rate capability and improved shelf life compared to zinc-carbon batteries. Urry’s innovations included replacing the Zinc case (Figure 1) with powdered Zinc, massively increasing the surface area, and improving discharge rate capability. He also replaced the acidic electrolyte with potassium hydroxide (KOH), further decreasing internal resistance and improving rate capability. Urry demonstrated his invention to his boss by racing two toy cars in the factory cafeteria, one with a traditional Zinc-carbon D cell, and another with his new battery. The first barely moved, while the second made a few trips along the length of the cafeteria.
Alkaline zinc cells are used in applications where the battery is used intermittently but needs to work reliably and is exposed to uncontrolled storage conditions, such as smoke alarms and watches.
Zinc silver-oxide batteries have high energy density, long shelf life, and flat voltage discharge profiles. They are commonly used in portable and miniature electronic applications such as watches, calculators, hearing aids, and toys. They use a zinc anode, a silver oxide cathode, and a KOH electrolyte when high power capability is required. A sodium hydrogide (NaOH) electrolyte is used if longer shelf life is desired. The high cost of silver mostly limits this chemistry to small batteries, except in space and military applications where cost is less important.
Why zinc silver-oxide over lithium-ion batteries for miniature electronic applications? The safety issues with pets or kids swallowing small cells that could get lodged in the esophagus presents a nightmare situation for a lithium chemistry. Further, owing to the low conductivity of organic electrolytes, lithium chemistry favors larger surface area for high rate capability applications.
Zinc air batteries are most commonly used in hearing aid applications, owing to their high-energy density, ideal voltage for the application, and long shelf life until activation. The battery chemistry uses a zinc anode, a potassium hydroxide electrolyte, and air as the cathode. The battery is activated by removing a sealing tab, and air is introduced into the cell. Using air instead of traditional cathode materials such as metal oxides enables manufacturing of smaller and lighter batteries. Disadvantages include sensitivity to the environment; once batteries are activated, they have to be used up quickly.
Lithium primary batteries: Lithium is the lightest metal in the periodic table and has a specific capacity of 3860 mAh/g compared to zinc at 820 mAh/g. Lithium also has an electrochemical reduction potential of 3.045 V against 0.76 V for zinc (i.e a lithium-based batteries provide a battery voltage of 3 V or greater). The combination of these two properties results in very high energy densities for lithium-based batteries.
However, lithium is highly reactive toward water and cannot be used with aqueous electrolytes unlike zinc. Organic electrolytes are commonly used, but these pale in conductivity compared to aqueous electrolytes like potassium hydroxide, zinc chloride, etc. and limit the power output of lithium batteries (in order to get power, low battery resistance and high electrolyte conductivity are required). On the plus side, the lower freezing points of organic electrolytes enables them to operate at lower temperatures than aqueous electrolyte-based battery systems.
Common Primary Lithium Batteries
Lithium manganese dioxide batteries use lithium metal as the anode and a manganese dioxide cathode. These are available in button cell and cylindrical formats. Because organic electrolytes have low conductivity, Li-MnO2 cell designs favor large surface area cell construction such as coin over button cells and jellyroll construction over the bobbin type to minimize internal resistance and enhance power capability. Lithium coin cells also are operated at lower currents than zinc silver oxide cells in order to minimize internal resistance and cell heating.
Bobbin Cells are optimized for energy.
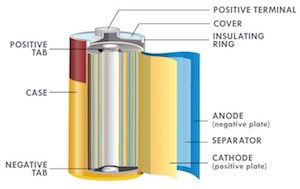
Jelly roll constructions are optimized for power.
Lithium iron sulfide batteries provide a higher energy density alternative to alkaline batteries at 1.5 V with superior performance at high drain rates, longer shelf life, better leak resistance, wider operating temperature range, and a reduction in weight. These cells are used in digital cameras and camcorders. Disadvantages include transportation restrictions due to the lithium metal content in the anode and the higher cost (each airline passenger is restricted to carrying 2 g of metallic lithium in primary batteries, or 8 g of rechargeable li-ion, which amounts to 2 lithium iron sulfide cells). These cells have a positive temperature coefficient (PTC) safety switch, which acts as a current limiter in case the cell overheats.
Battery cell chemistry dictates many of the cell properties that impact battery performance, thereby making it a key consideration in battery selection. As a follow up to the primary battery cell chemistry we explored in this blog, our next post will delve into secondary battery chemistries.
Interested in improving your battery reliability? Request a quote here.