借助工程仿真加速医疗器械创新
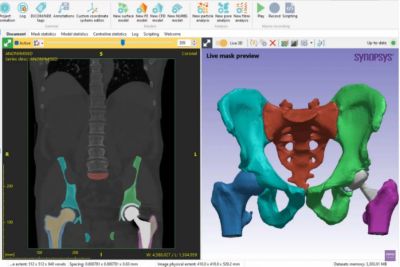
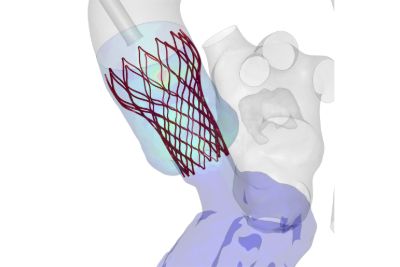
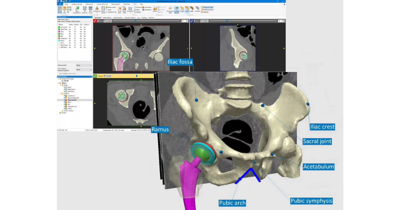
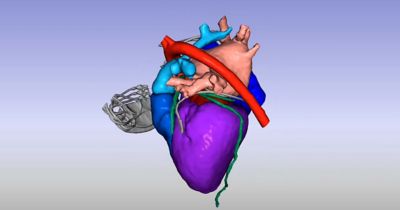
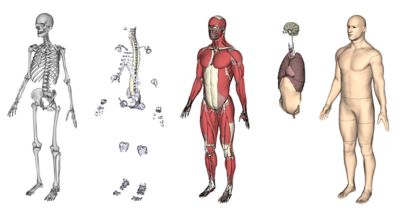
无论是用于挽救生命还是提高生活质量,医疗器械都需要耐受人体的严苛使用环境,在其预期使用寿命内按设计正常运行,并且不干扰人体的正常生理功能。新思科技旗下Ansys提供了计算机建模和仿真(CM&S)、人工智能(AI)以及数字孪生等所需的计算机仿真(In Silico)技术,以帮助医疗器械行业为患者提供安全、创新的设备。通过以虚拟方式测试医疗器械,制造商可以迭代和优化设计,并确定它们在人体内部的相互作用方式,同时测试生物相容性、MRI安全性、疲劳性能、血液损伤等。
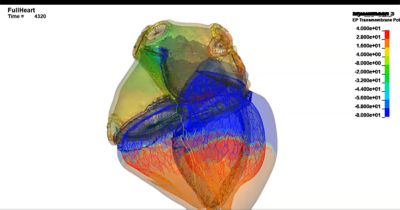
借助计算机仿真(In Silico)解决方案对抗心血管疾病
应用
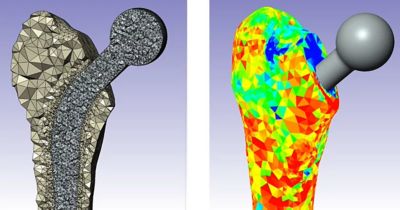
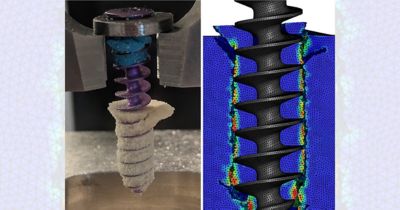
仿真提供了一种变革性的方法,可最大限度地降低各种医疗应用中的开发风险和成本。通过虚拟测试,制造商可以尽早识别设计缺陷,从而减少对成本高昂的物理原型和广泛测试阶段的依赖。
精选解决方案
真实世界仿真的实际应用
以下展示了我们精选的全球同仁、客户和合作伙伴使用仿真推进医疗器械发展的成功案例和前沿洞见。
现在就开始行动吧!
如果您面临工程方面的挑战,我们的团队将随时为您提供帮助。我们拥有丰富的经验并秉持创新承诺,期待与您联系。让我们携手合作,将您的工程挑战转化为价值增长和成功的机遇。欢迎立即联系我们进行交流。