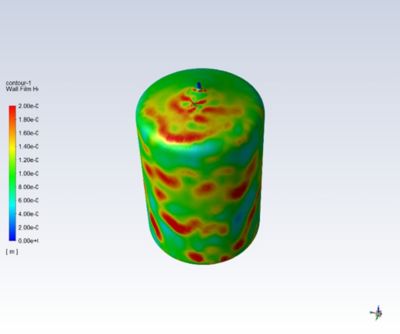
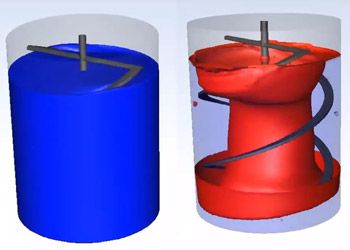
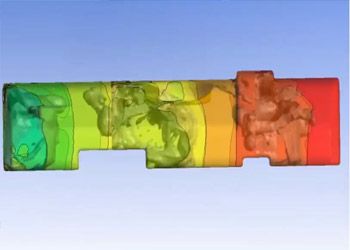
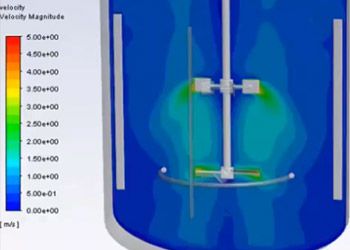
Ninety-six percent of the top 50 healthcare companies in the world are using engineering simulation and computer-based models routinely. Leading medical device companies are realizing that their engineers should be using simulation regularly. The FDA and other regulatory authorities encourage the adoption of computer models and simulation — also known as the “in silico” approach — to accelerate the approval process, but many companies are still unsure of how best to adopt and deploy this technology.
This monthly webinar series shares the knowledge and experience of Ansys experts and our partners to guide you in the strategic adoption of engineering simulation.